Customer Pain Points
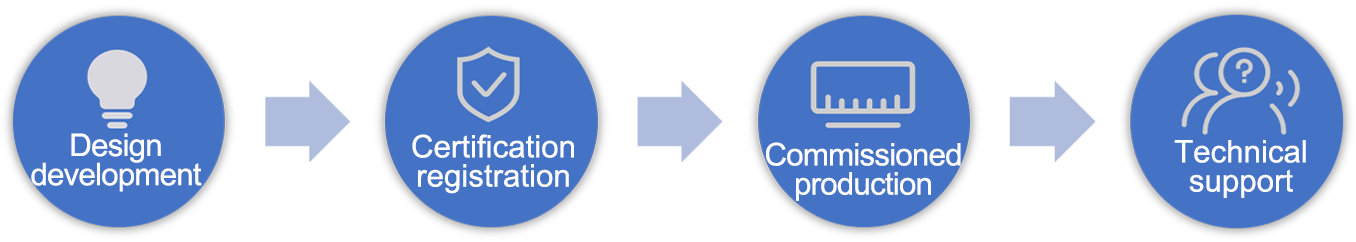
One-stop Service
CDMO Series
• Has a standardized and scientific quality management system, certified by ISO 9001, ISO 13485, ISO 14001, and ISO 45001.




• Has over 6000 square meters of professional medical instrument production workshop.
• Rich R&D experience in the field of medical devices and a complete customization process.
Provides compliant CDMO/OEM one-stop services in the fields of immunodiagnostics, molecular diagnostics, and immunohistochemistry according to the "Regulations on the Supervision and Administration of Medical Device Production".










